

CORPORATE PRESENTATION March 2019 NASDAQ: XOMA A Royalty Aggregation Company Exhibit 99.1

DISCLAIMERS Certain statements in this presentation are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, including statements regarding: future potential monetization opportunities, active transactions with significant financial implications, collaborations poised for significant financial contribution, our library of potentially value-generating assets, future potential for milestone and royalty payments, the potential of our antibody discovery engine, potential out-licensing of our internal compounds and products, the ability of our partners and their licensees to successfully develop their pipeline programs, the productivity of acquired assets, our revenue forecasts, upcoming internal milestones and value catalysts, our future cash needs, our strategy for value creation, and other statements that relate to future periods. These statements are not guarantees of future performance and undue reliance should not be placed on them. They are based on assumptions that may not prove accurate, and actual results could differ materially from those anticipated due to certain risks inherent in the biotechnology industry and for companies engaged in the development of new products in a regulated market. Potential risks to XOMA meeting these expectations are described in more detail in XOMA's most recent filing on Form 10-K and in other SEC filings. Consider such risks carefully when considering XOMA's prospects. Any forward-looking statements represent XOMA’s views only as of the date of this presentation and should not be relied upon as representing its views as of any subsequent date. XOMA disclaims any obligation to update any forward-looking statement, except as required by law. NOTE: All references to “portfolio” in this presentation are to milestone and/or royalty rights associated with a basket of drug products in development. All references to “assets” in this presentation are to milestone and/or royalty rights associated with individual drug product candidates in development.
Monetizing and aggregating pre-commercial drug royalties Use portfolio approach to expand number of royalty positions Differentiate by focusing on development-stage assets with blockbuster potential licensed to large-cap partners Providing exposure, through royalties, to the upside potential of biotech Capital-efficient model where R&D costs are borne by partners Cash inflows from interim milestone payments Exposure risk mitigated through portfolio effects Expected value appreciation driven by: Assets advancing in hands of partners Acquiring additional assets to expand revenue potential and further mitigate risk Portfolio of 45 assets today and growing XOMA SNAPSHOT
XOMA’s Approach Accumulate and hold milestone and royalty interests in multiple assets partnered with pharmaceutical companies who conduct and fund R&D. XOMA’S PORTFOLIO MODEL Traditional Biotech Companies Fund and perform internal research and development; expensive and can take up to 15 years. Many programs fail but those that succeed can be incredibly valuable.
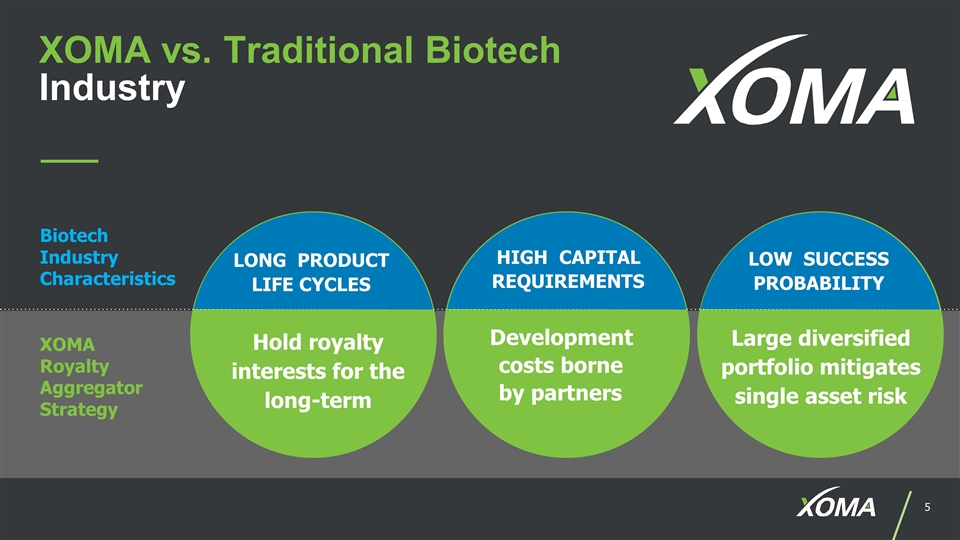
XOMA vs. Traditional Biotech Industry Biotech Industry Characteristics Hold royalty interests for the long-term Development costs borne by partners Large diversified portfolio mitigates single asset risk XOMA Royalty Aggregator Strategy LONG PRODUCT LIFE CYCLES HIGH CAPITAL REQUIREMENTS LOW SUCCESS PROBABILITY
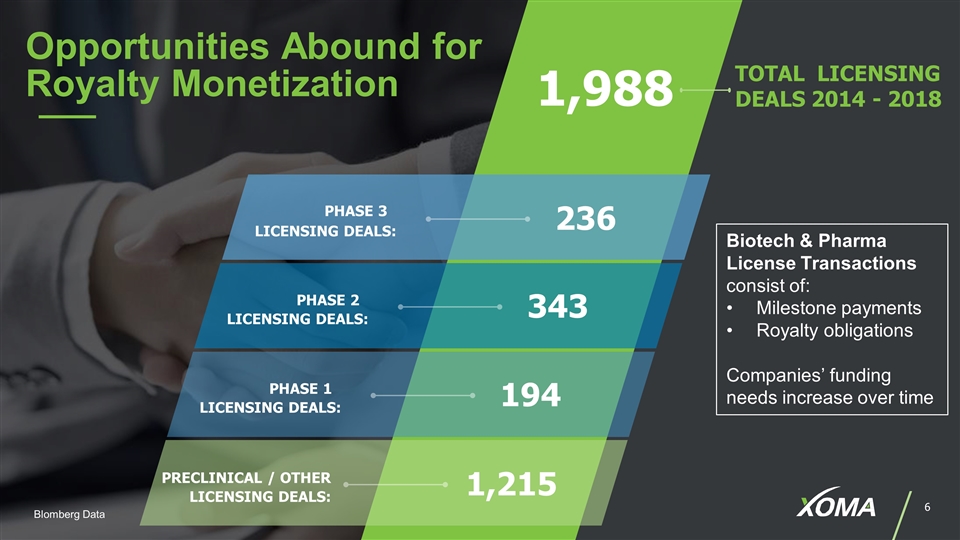
PHASE 3 LICENSING DEALS: 236 TOTAL LICENSING DEALS 2014 - 2018 1,988 Opportunities Abound for Royalty Monetization PHASE 2 LICENSING DEALS: 343 PHASE 1 LICENSING DEALS: 194 PRECLINICAL / OTHER LICENSING DEALS: 1,215 Biotech & Pharma License Transactions consist of: Milestone payments Royalty obligations Companies’ funding needs increase over time Blomberg Data
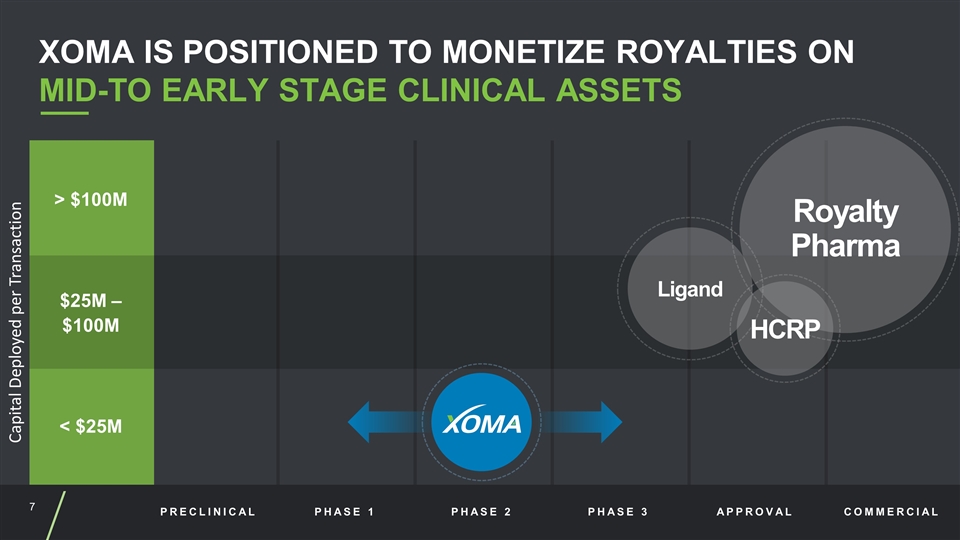
PRECLINICAL PHASE 1 PHASE 2 PHASE 3 APPROVAL COMMERCIAL > $100M $25M – $100M < $25M Capital Deployed per Transaction Ligand Royalty Pharma HCRP XOMA IS POSITIONED TO MONETIZE ROYALTIES ON MID-TO EARLY STAGE CLINICAL ASSETS
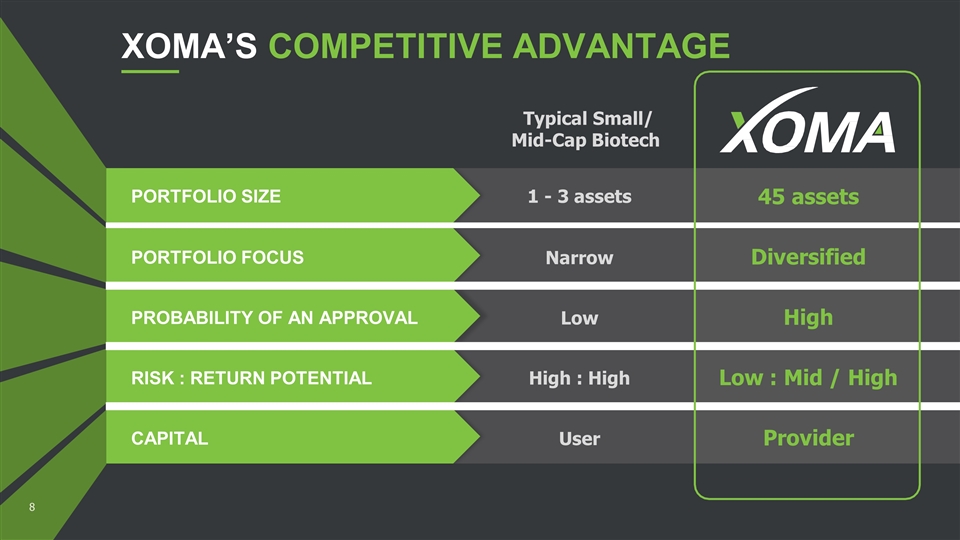
XOMA’S COMPETITIVE ADVANTAGE Typical Small/ Mid-Cap Biotech PORTFOLIO SIZE 1 - 3 assets 45 assets PORTFOLIO FOCUS Narrow Diversified PROBABILITY OF AN APPROVAL Low High RISK : RETURN POTENTIAL High : High Low : Mid / High CAPITAL User Provider
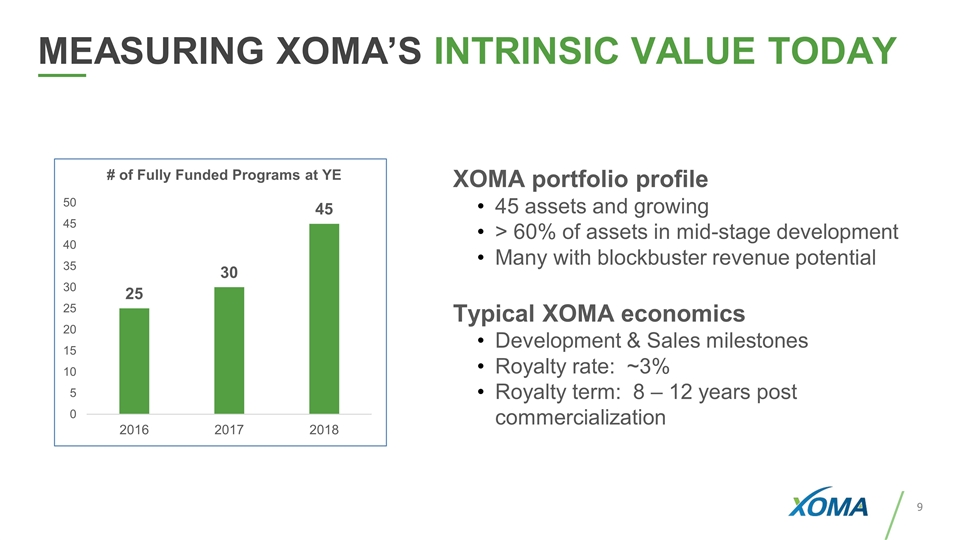
MEASURING XOMA’S INTRINSIC VALUE TODAY XOMA portfolio profile 45 assets and growing > 60% of assets in mid-stage development Many with blockbuster revenue potential Typical XOMA economics Development & Sales milestones Royalty rate: ~3% Royalty term: 8 – 12 years post commercialization

26 XOMA’S MODEL: HOLD & BUY MORE TOTAL: HOLD LEGACY ASSETS Allow current portfolio of assets to advance, fully funded by Partners; hold milestone & royalty payment rights BUY MORE & HOLD Acquire rights to additional potential milestone & royalty assets 45 ASSETS 38 ASSETS 7 ASSETS
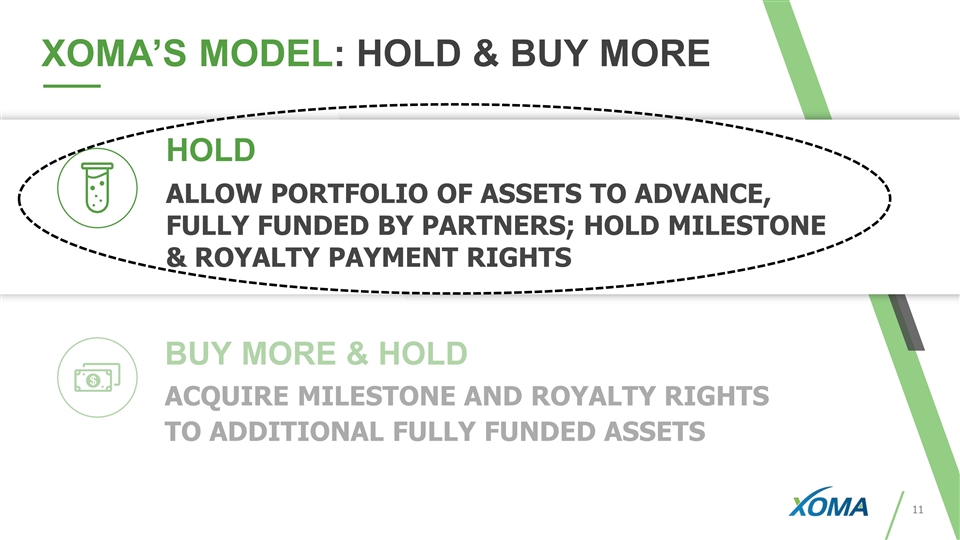
BUY MORE & HOLD ACQUIRE MILESTONE AND ROYALTY RIGHTS TO ADDITIONAL FULLY FUNDED ASSETS XOMA’S MODEL: HOLD & BUY MORE HOLD ALLOW PORTFOLIO OF ASSETS TO ADVANCE, FULLY FUNDED BY PARTNERS; HOLD MILESTONE & ROYALTY PAYMENT RIGHTS
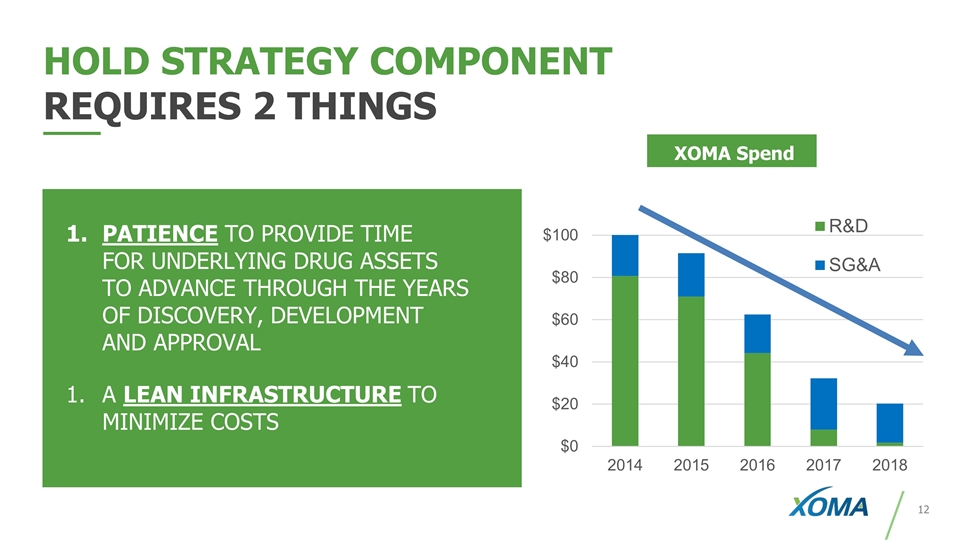
HOLD STRATEGY COMPONENT REQUIRES 2 THINGS XOMA Spend PATIENCE TO PROVIDE TIME FOR UNDERLYING DRUG ASSETS TO ADVANCE THROUGH THE YEARS OF DISCOVERY, DEVELOPMENT AND APPROVAL A LEAN INFRASTRUCTURE TO MINIMIZE COSTS
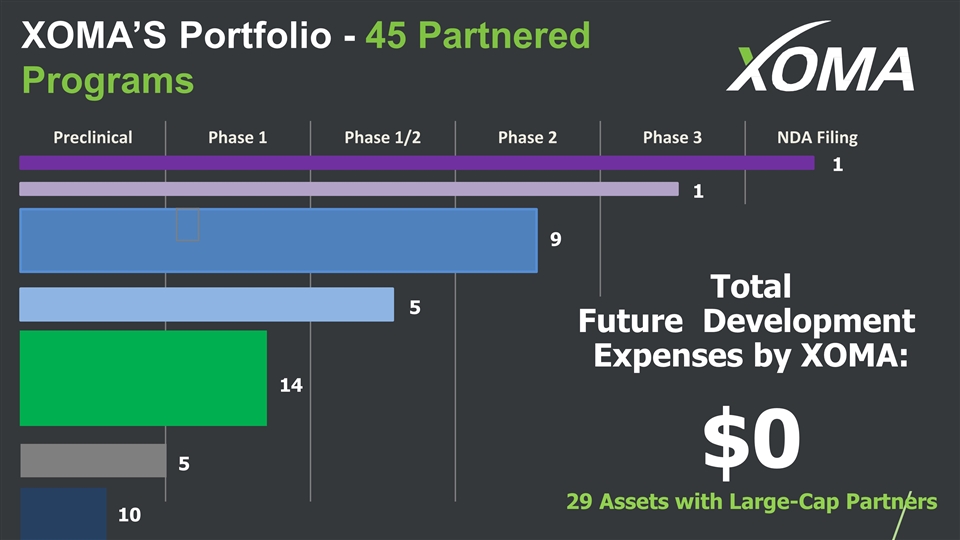
XOMA’S Portfolio - 45 Partnered Programs Preclinical Phase 1 Phase 1/2 Phase 2 Phase 3 NDA Filing Total Future Development Expenses by XOMA: $0 29 Assets with Large-Cap Partners 1 1 9 14 10 5 5
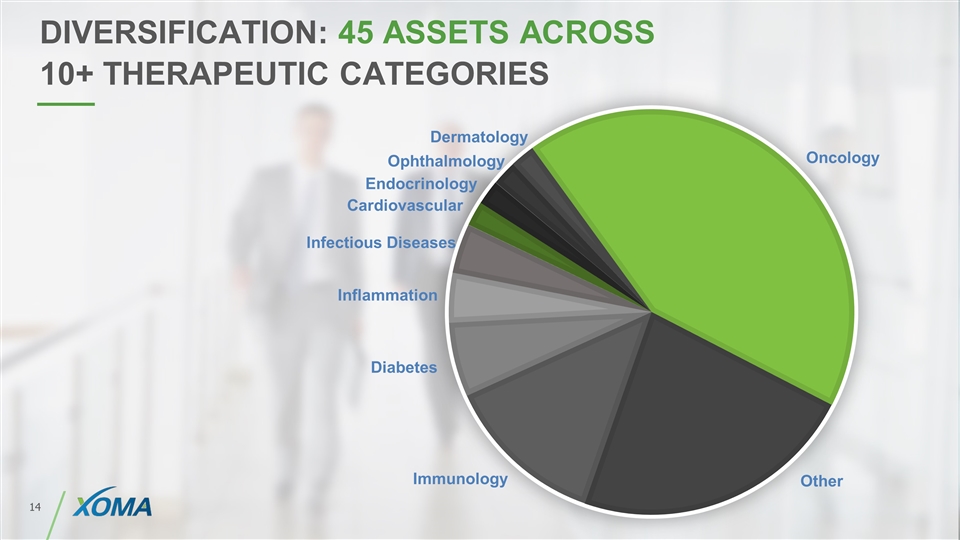
DIVERSIFICATION: 45 ASSETS ACROSS 10+ THERAPEUTIC CATEGORIES Oncology Other Immunology Diabetes Inflammation Infectious Diseases Cardiovascular Endocrinology Ophthalmology Dermatology
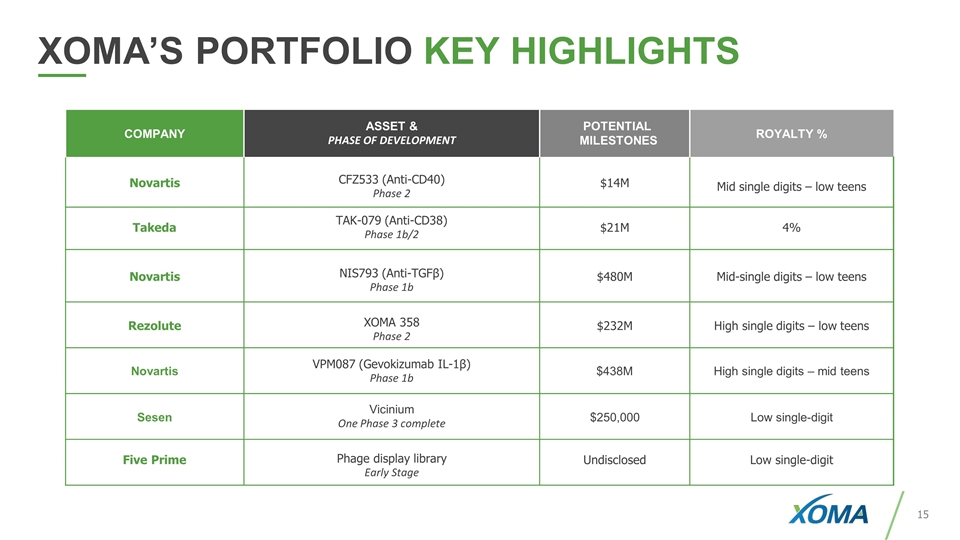
COMPANY ASSET & PHASE OF DEVELOPMENT POTENTIAL MILESTONES ROYALTY % Novartis CFZ533 (Anti-CD40) Phase 2 $14M Mid single digits – low teens Takeda TAK-079 (Anti-CD38) Phase 1b/2 $21M 4% Novartis NIS793 (Anti-TGFβ) Phase 1b $480M Mid-single digits – low teens Rezolute XOMA 358 Phase 2 $232M High single digits – low teens Novartis VPM087 (Gevokizumab IL-1β) Phase 1b $438M High single digits – mid teens Sesen Vicinium One Phase 3 complete $250,000 Low single-digit Five Prime Phage display library Early Stage Undisclosed Low single-digit XOMA’S PORTFOLIO KEY HIGHLIGHTS
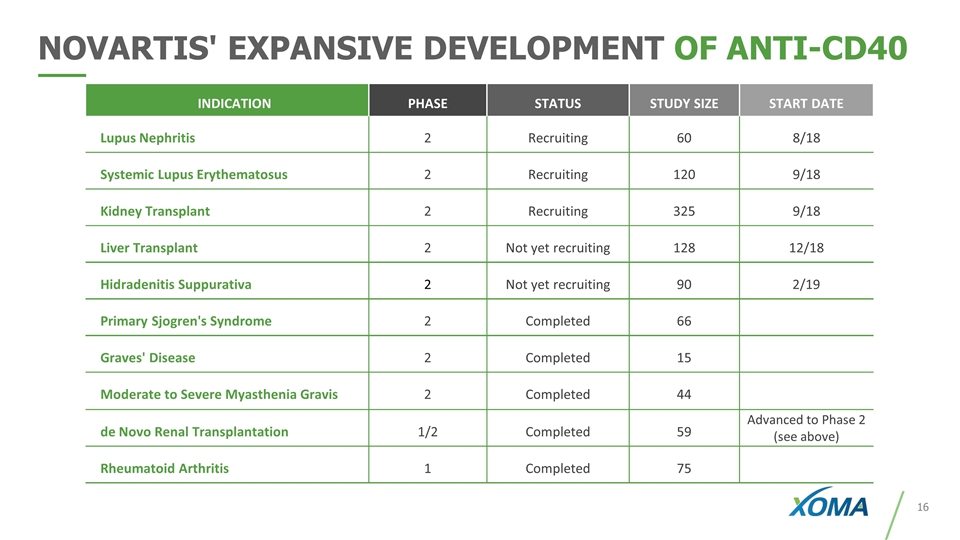
INDICATION PHASE STATUS STUDY SIZE START DATE Lupus Nephritis 2 Recruiting 60 8/18 Systemic Lupus Erythematosus 2 Recruiting 120 9/18 Kidney Transplant 2 Recruiting 325 9/18 Liver Transplant 2 Not yet recruiting 128 12/18 Hidradenitis Suppurativa 2 Not yet recruiting 90 2/19 Primary Sjogren's Syndrome 2 Completed 66 Graves' Disease 2 Completed 15 Moderate to Severe Myasthenia Gravis 2 Completed 44 de Novo Renal Transplantation 1/2 Completed 59 Advanced to Phase 2 (see above) Rheumatoid Arthritis 1 Completed 75 NOVARTIS' EXPANSIVE DEVELOPMENT OF ANTI-CD40
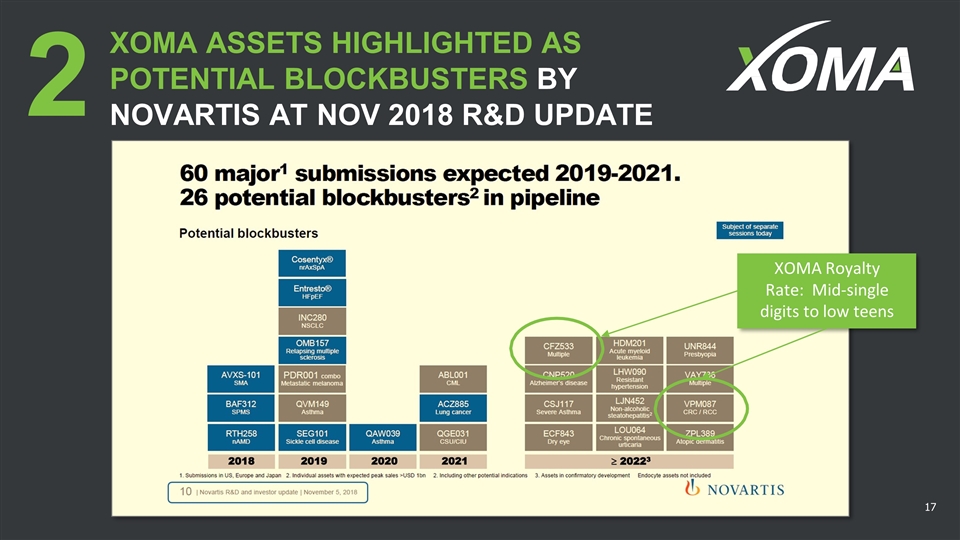
2 XOMA ASSETS HIGHLIGHTED AS POTENTIAL BLOCKBUSTERS BY NOVARTIS AT NOV 2018 R&D UPDATE XOMA Royalty Rate: Mid-single digits to low teens
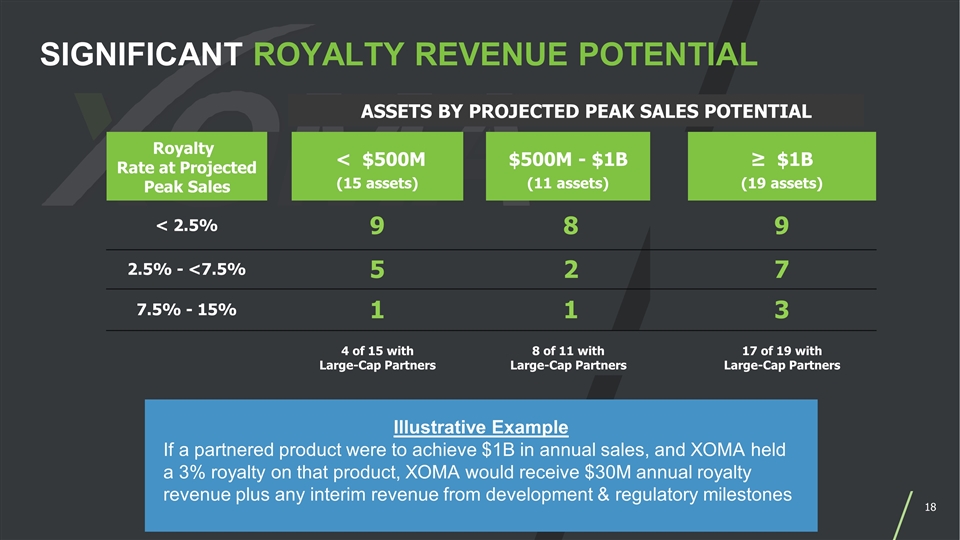
Royalty Rate at Projected Peak Sales < $500M (15 assets) $500M - $1B (11 assets) ≥ $1B (19 assets) < 2.5% 9 8 9 2.5% - <7.5% 5 2 7 7.5% - 15% 1 1 3 4 of 15 with Large-Cap Partners 8 of 11 with Large-Cap Partners 17 of 19 with Large-Cap Partners ASSETS BY PROJECTED PEAK SALES POTENTIAL SIGNIFICANT ROYALTY REVENUE POTENTIAL Illustrative Example If a partnered product were to achieve $1B in annual sales, and XOMA held a 3% royalty on that product, XOMA would receive $30M annual royalty revenue plus any interim revenue from development & regulatory milestones
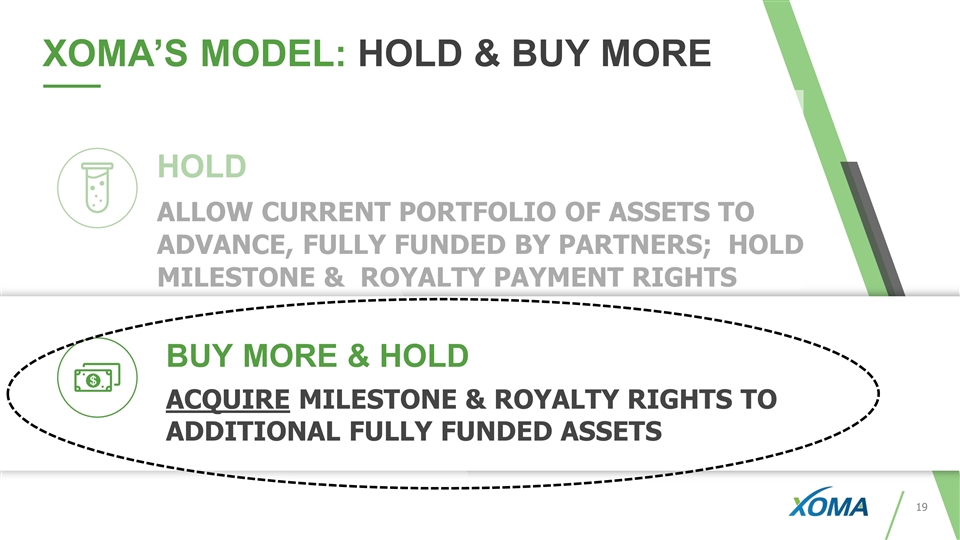
BUY MORE & HOLD ACQUIRE MILESTONE & ROYALTY RIGHTS TO ADDITIONAL FULLY FUNDED ASSETS XOMA’S MODEL: HOLD & BUY MORE HOLD ALLOW CURRENT PORTFOLIO OF ASSETS TO ADVANCE, FULLY FUNDED BY PARTNERS; HOLD MILESTONE & ROYALTY PAYMENT RIGHTS
XOMA ACQUISITION STRATEGY IS DISTINCT Acquire milestone and royalty licenses to high-potential, fully funded assets Focus on mid-clinical stage assets Partner companies pay all development costs Ever-increasing pipeline of potential targets Team entirely focused on acquiring new royalty assets THE BENEFITS TO ASSET SELLERS Recognize value of non-dilutive, non-recourse financing Few options to monetize license agreements associated with partnered mid-stage clinical assets Immediate cash infusion to advance high-priority internal programs to improve human health
KEY ATTRIBUTES OF XOMA TARGET ASSETS PRE-COMMERCIAL THERAPEUTIC ASSETS Phase 1, 2, or 3 LONG DURATION OF MARKET EXCLUSIVITY Patent expiration or regulatory exclusivity HIGH REVENUE POTENTIAL High unmet need or clear clinical benefit over alternatives STRONG DEVELOPER/MARKETER Assets partnered with high-quality pharma / biopharma companies Rx
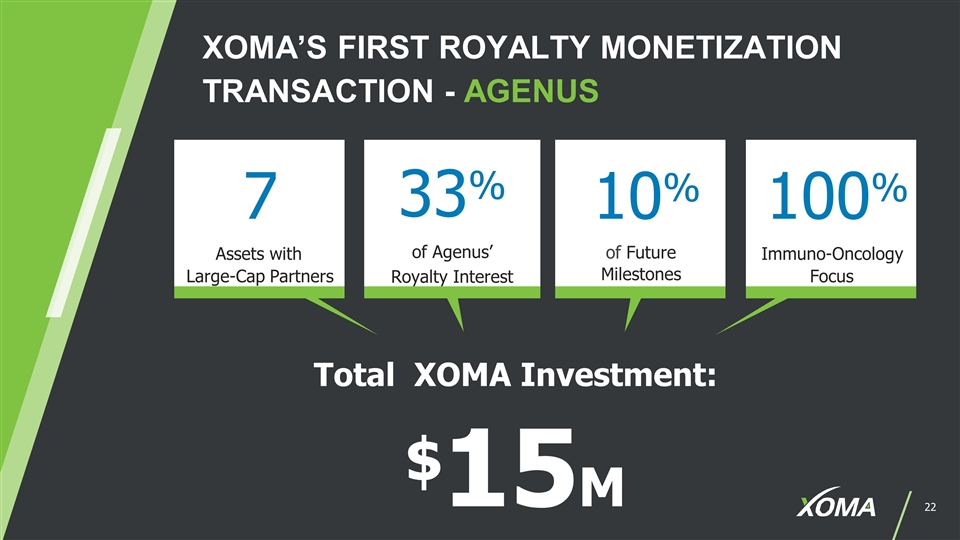
XOMA’S FIRST ROYALTY MONETIZATION TRANSACTION - AGENUS Total XOMA Investment: $15M 7 Assets with Large-Cap Partners 33% of Agenus’ Royalty Interest 10% 100% Immuno-Oncology Focus of Future Milestones
AGENUS TRANSACTION HITS ALL KEY ATTRIBUTES OF XOMA TARGET ASSETS LONG DURATION EXCLUSIVITY HIGH REVENUE POTENTIAL STRONG DEVELOPER/MARKETER Rx PRE-COMMERCIAL THERAPEUTIC ASSETS 7 total assets; 5 in clinical development Potentially 10-years post commercialization Potentially important immuno-oncology drugs Merck, Incyte

26 XOMA’S MODEL: HOLD & BUY MORE TOTAL: HOLD LEGACY ASSETS Allow current portfolio of assets to advance, fully funded by Partners; hold milestone & royalty payment rights BUY MORE & HOLD Acquire rights to additional potential milestone & royalty assets 45 ASSETS 38 ASSETS 7 ASSETS

RECENT HIGHLIGHTS $20M Rights Offering led by BVF Partners Established $20M LoC with Silicon Valley Bank Acquired milestone & royalty interests in 6 Incyte assets & 1 Merck asset from Agenus for $15M Added $5.5M to the balance sheet in Feb ’19 from Rezolute milestone Added Barbara Kosacz, Partner at Cooley LLP, to Board of Directors Maintained lean cost infrastructure and financial discipline Operational Novartis announced gevokizumab will enter oncology clinical studies Sesen Bio disclosed positive top-line Phase 3 data on Vicinium – Jan ’19 Novartis / CFZ533 progress Initiated Phase 2 trials: Kidney Transplant Rejection Systemic Lupus Erythematosus Additional Phase 2 trials planned: Liver Transplant Rejection Hidradenitis Suppurativa Partners & Partnered Assets
LOOKING AHEAD Acquire additional milestone and royalty interest assets to take portfolio to >50 Maintain lean cost infrastructure and financial discipline Current balance sheet sufficient to fund operations for multiple years $1M per month core G&A expense Operational Novartis – gevokizumab Phase 1b study – first patient dosing Novartis – TGFβ advancing to Phase 2 Incyte – Immuno-oncology programs – data readouts from Phase 1/2 and Phase 1 Merck – Immuno-oncology program – data readout from Phase 1 Sesen Bio – Full data readout from Phase 3 Vicinium study Partners & Partnered Assets
Why is XOMA’s portfolio valuable? XOMA holds 45 current assets; pharmaceutical partners fund the research & development and cover 100% of costs XOMA sources royalty rights through deep industry network XOMA constructs an increasingly diverse and expanding portfolio to increase odds of success and mitigate binary risk XOMA has low-cost infrastructure; future potential revenues largely fall to bottom line