

corporate PRESENTATION December 2020 NASDAQ: XOMA A Royalty Aggregation Company Exhibit 99.1

DISCLAIMERS Certain statements in this presentation are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934, including statements regarding: future potential monetization opportunities, active transactions with significant financial implications, collaborations poised for significant financial contribution, our library of potentially value-generating assets, future potential for milestone and royalty payments, the potential of our antibody discovery engine, potential out-licensing of our internal compounds and products, the ability of our partners and their licensees to successfully develop their pipeline programs, the productivity of acquired assets, our revenue forecasts, upcoming internal milestones and value catalysts, our future cash needs, our strategy for value creation, and other statements that relate to future periods. These statements are not guarantees of future performance and undue reliance should not be placed on them. They are based on assumptions that may not prove accurate, and actual results could differ materially from those anticipated due to certain risks inherent in the biotechnology industry and for companies engaged in the development of new products in a regulated market. Potential risks to XOMA meeting these expectations are described in more detail in XOMA's most recent filing on Form 10-K and in other SEC filings. Consider such risks carefully when considering XOMA's prospects. Any forward-looking statements represent XOMA’s views only as of the date of this presentation and should not be relied upon as representing its views as of any subsequent date. XOMA disclaims any obligation to update any forward-looking statement, except as required by law. NOTE: All references to “portfolio” in this presentation are to milestone and/or royalty rights associated with a basket of drug products in development. All references to “assets” in this presentation are to milestone and/or royalty rights associated with individual drug product candidates in development. References to royalties or royalty rates contained herein refer to future potential payment streams regardless of whether or not they are technically defined as royalties in the underlying contractual agreement; further, any rates referenced herein are subject to potential future contractual adjustments.

Acquire pre-commercial drug royalties Use portfolio approach to expand number of royalty positions Differentiate by focusing on development-stage assets with blockbuster potential licensed to large-cap partners Provide exposure, through royalties, to the upside potential of biotech Capital-efficient model where R&D costs are borne by partners Cash inflows from interim milestone payments Exposure risk mitigated through portfolio effects Expected value appreciation driven by: Advancement of assets by partners who spend hundreds of millions of dollars to develop XOMA royalty assets Acquisition of additional assets by XOMA to expand revenue potential and further mitigate risk Portfolio of 65+ assets in >30 disclosed indications today and growing XOMA SNAPSHOT
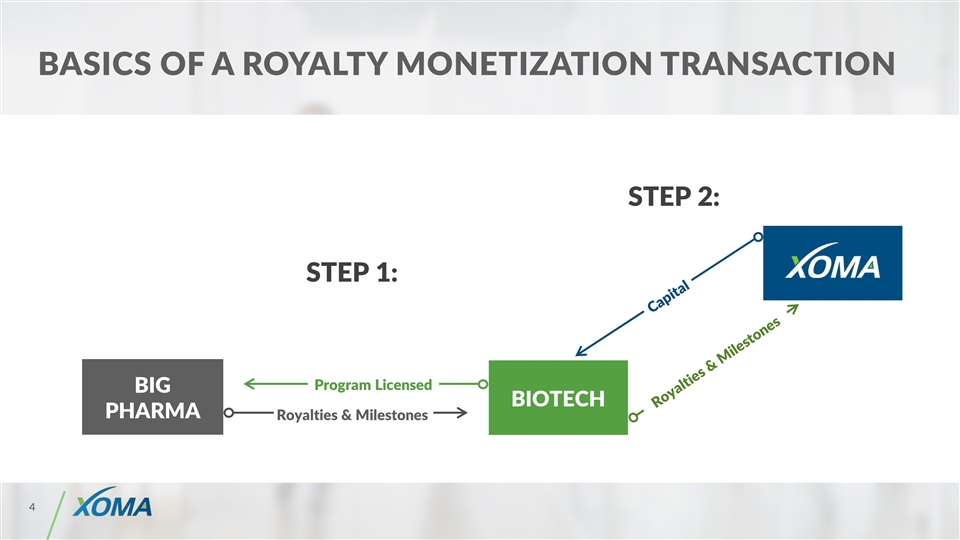
Basics of a royalty monetization TRANSACTION BIOTECH BIG PHARMA Capital Royalties & Milestones Royalties & Milestones STEP 1: Program Licensed STEP 2:

Royalty Financings can Help Companies Raise Capital More Efficiently than Equity and is Less Onerous than Debt Equity Debt Royalty Financing Cost of Capital High Medium to High Low to Medium Dilution High Low NA Covenants/Restrictions Medium High Low Transaction Cost High Medium to High Low Control High Low to Medium NA Diligence/Disruption High Medium to High Low Collateral N/A All Assets Limited to Royalty Asset(s) Equity Royalty Financing Debt COST OF CAPITAL
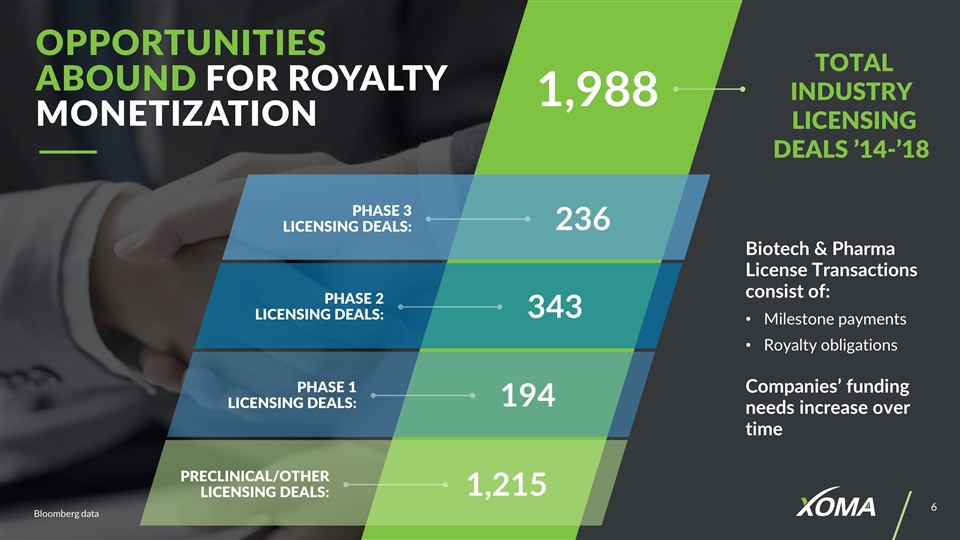
236 1,988 343 194 1,215 Biotech & Pharma License Transactions consist of: Milestone payments Royalty obligations Companies’ funding needs increase over time Bloomberg data PHASE 3 Licensing Deals: PHASE 2 Licensing Deals: PHASE 1 Licensing Deals: Preclinical/other Licensing Deals: Opportunities Abound for Royalty Monetization Total Industry Licensing Deals ’14-’18
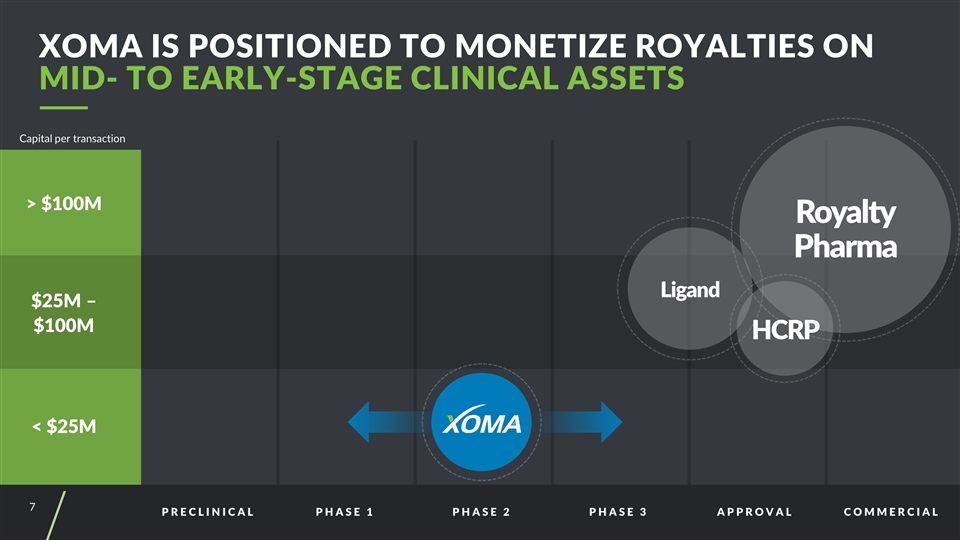
PRECLINICAL PHASE 1 PHASE 2 PHASE 3 APPROVAL COMMERCIAL > $100M $25M – $100M < $25M Capital per transaction Ligand Royalty Pharma HCRP XOMA is Positioned to Monetize Royalties on MID- TO EARLY-STAGE CLINICAL ASSETS
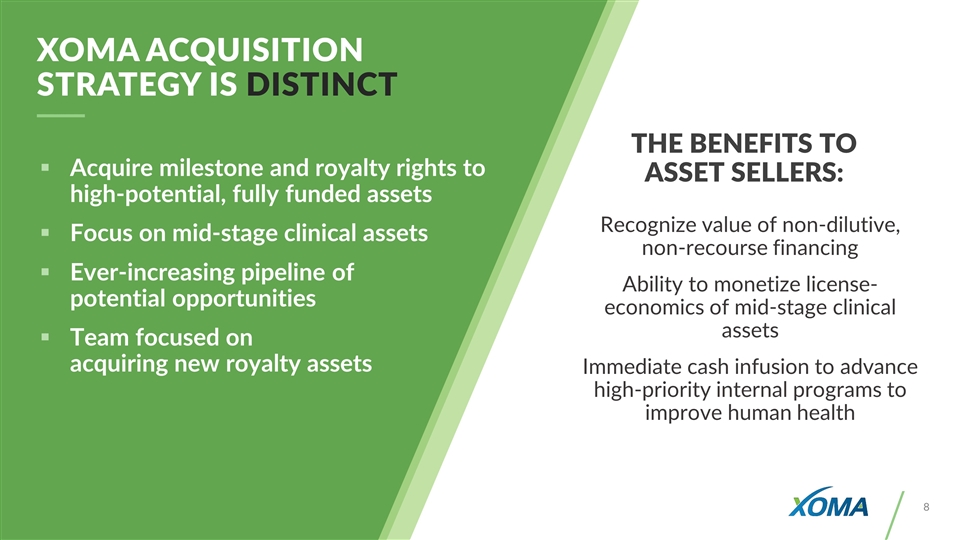
THE BENEFITS TO ASSET SELLERS: Recognize value of non-dilutive, non-recourse financing Ability to monetize license- economics of mid-stage clinical assets Immediate cash infusion to advance high-priority internal programs to improve human health Acquire milestone and royalty rights to high-potential, fully funded assets Focus on mid-stage clinical assets Ever-increasing pipeline of potential opportunities Team focused on acquiring new royalty assets XOMA ACQUISITION STRATEGY IS DISTINCT
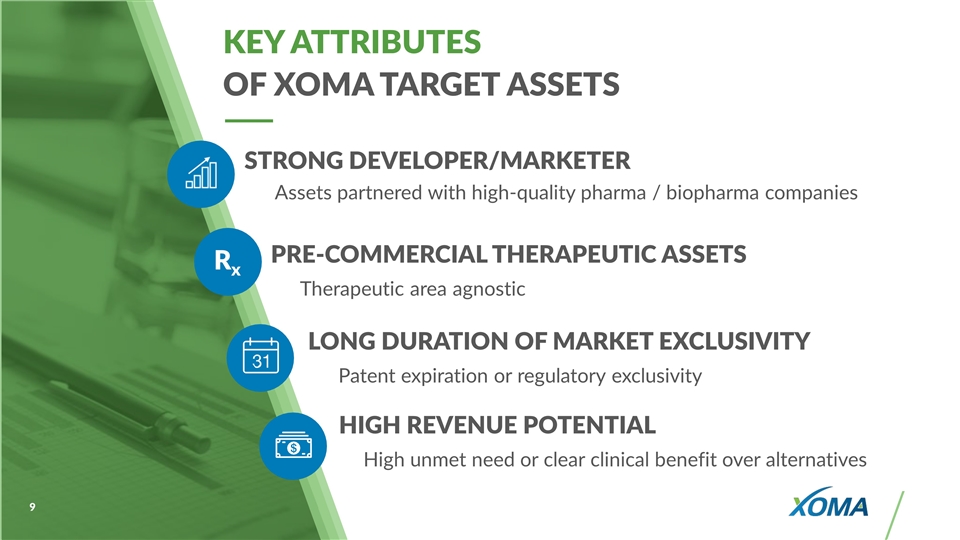
KEY ATTRIBUTES OF XOMA TARGET ASSETS PRE-COMMERCIAL THERAPEUTIC ASSETS Therapeutic area agnostic LONG DURATION OF MARKET EXCLUSIVITY Patent expiration or regulatory exclusivity Rx STRONG DEVELOPER/MARKETER Assets partnered with high-quality pharma / biopharma companies HIGH REVENUE POTENTIAL High unmet need or clear clinical benefit over alternatives
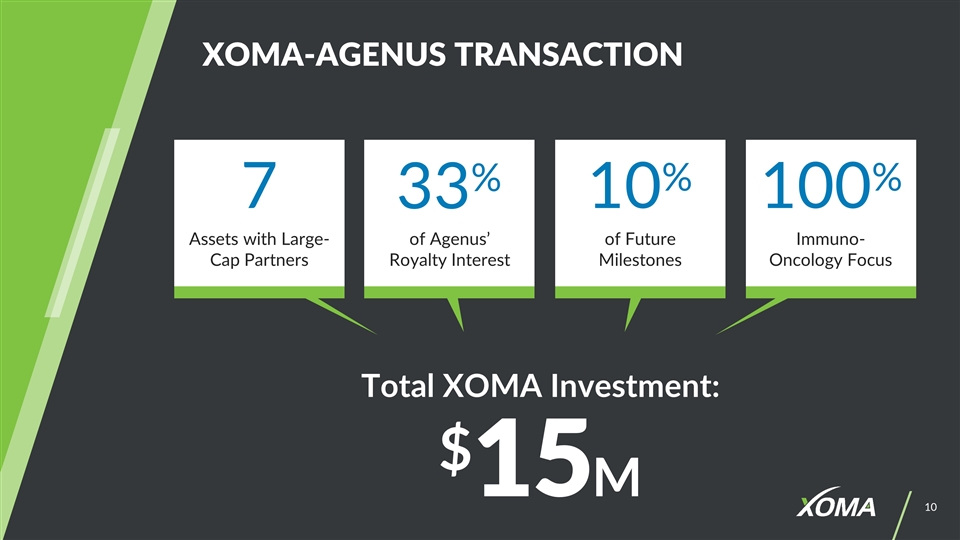
XOMA-AGENUS TRANSACTION Total XOMA Investment: $15M 7 33% 10% 100% of Future Milestones Assets with Large-Cap Partners of Agenus’ Royalty Interest Immuno- Oncology Focus
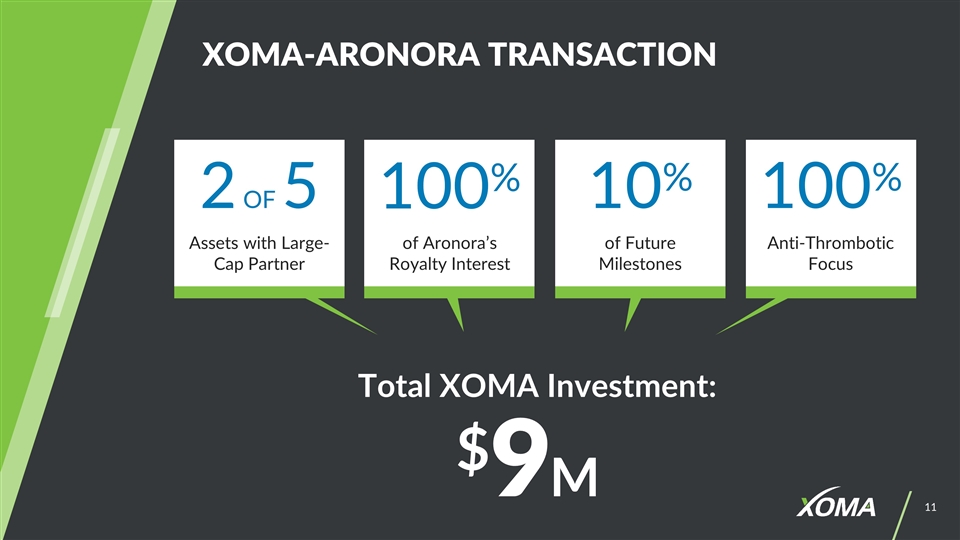
XOMA-ARONORA TRANSACTION Total XOMA Investment: $9M 2 OF 5 100% 10% 100% of Future Milestones Assets with Large-Cap Partner of Aronora’s Royalty Interest Anti-Thrombotic Focus
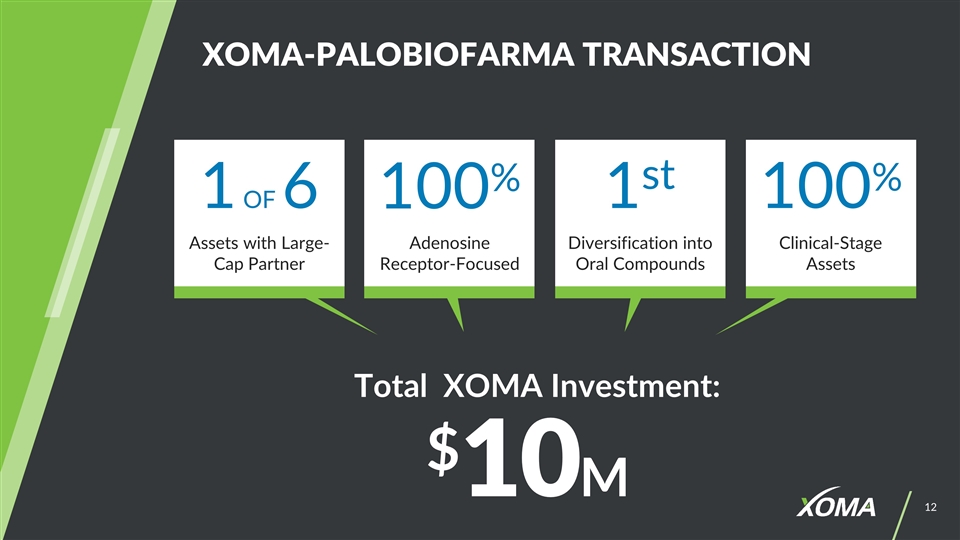
XOMA-PALOBIOFARMA TRANSACTION Total XOMA Investment: $10M 1 OF 6 100% 1st 100% Diversification into Oral Compounds Assets with Large-Cap Partner Adenosine Receptor-Focused Clinical-Stage Assets
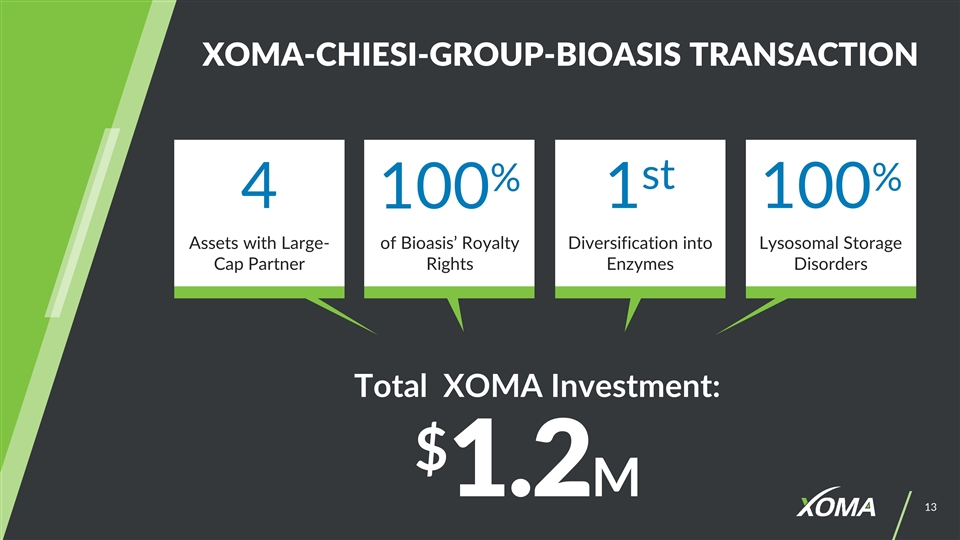
XOMA-CHIESI-GROUP-BIOASIS TRANSACTION Total XOMA Investment: $1.2M 4 100% 1st 100% Diversification into Enzymes Assets with Large-Cap Partner of Bioasis’ Royalty Rights Lysosomal Storage Disorders
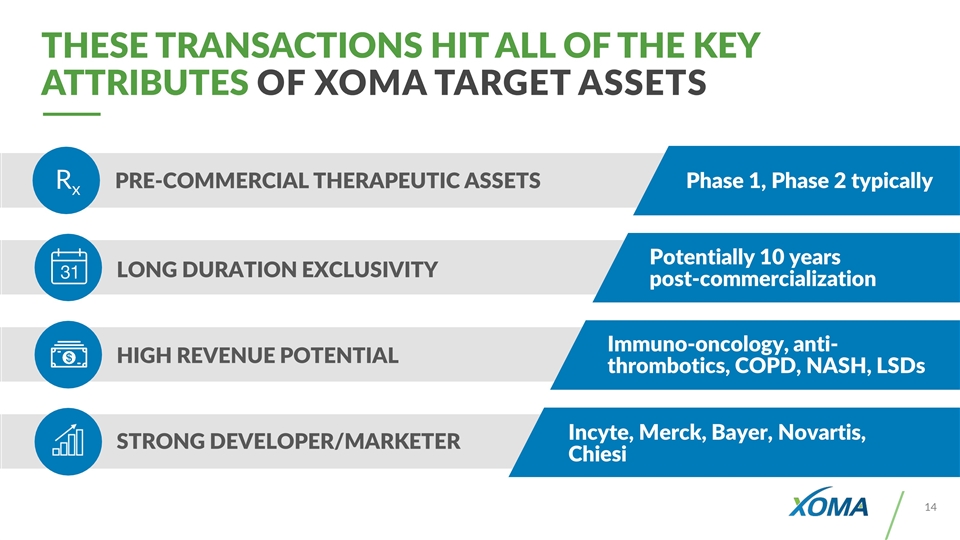
LONG DURATION EXCLUSIVITY HIGH REVENUE POTENTIAL STRONG DEVELOPER/MARKETER Rx PRE-COMMERCIAL THERAPEUTIC ASSETS Phase 1, Phase 2 typically Potentially 10 years post-commercialization Immuno-oncology, anti-thrombotics, COPD, NASH, LSDs Incyte, Merck, Bayer, Novartis, Chiesi THESE TRANSACTIONS HIT ALL OF THE KEY ATTRIBUTES OF XOMA TARGET ASSETS
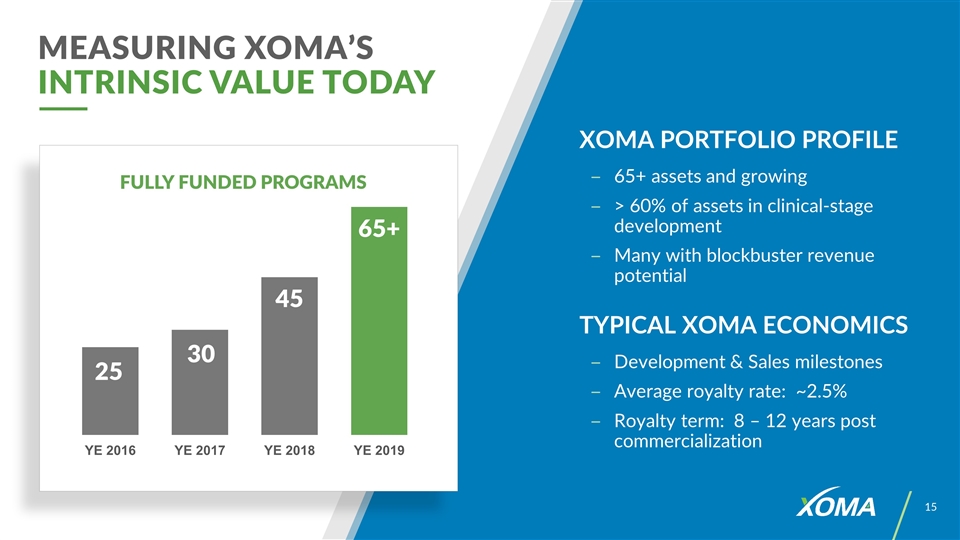
XOMA PORTFOLIO PROFILE 65+ assets and growing > 60% of assets in clinical-stage development Many with blockbuster revenue potential TYPICAL XOMA ECONOMICS Development & Sales milestones Average royalty rate: ~2.5% Royalty term: 8 – 12 years post commercialization MEASURING XOMA’S INTRINSIC VALUE TODAY FULLY FUNDED PROGRAMS
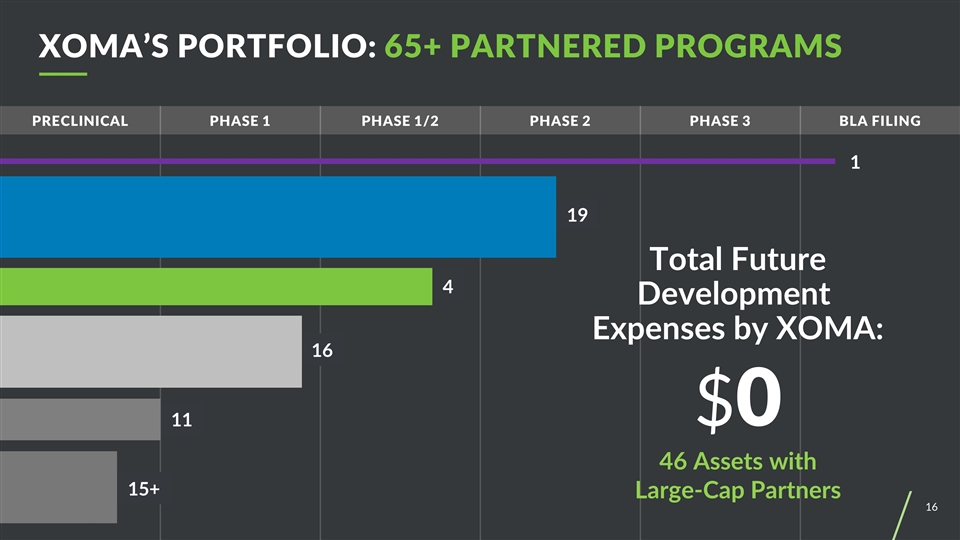
Preclinical PHASE 1 Phase 1/2 Phase 2 Phase 3 BLA filing 1 Total Future Development Expenses by XOMA: $0 46 Assets with Large-Cap Partners 19 4 16 11 15+ XOMA’S PORTFOLIO: 65+ PARTNERED PROGRAMS
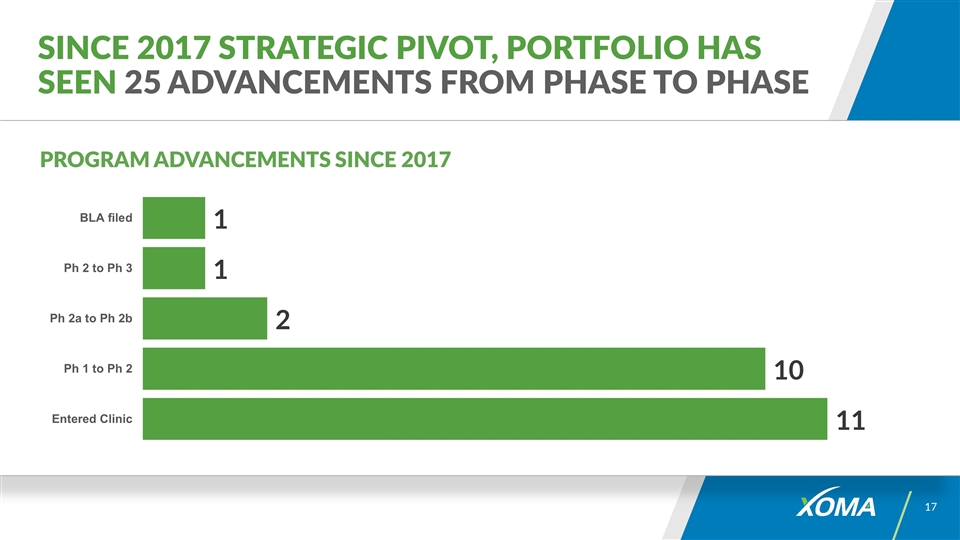
Since 2017 Strategic Pivot, Portfolio has seen 25 Advancements from Phase to Phase PROGRAM ADVANCEMENTS SINCE 2017

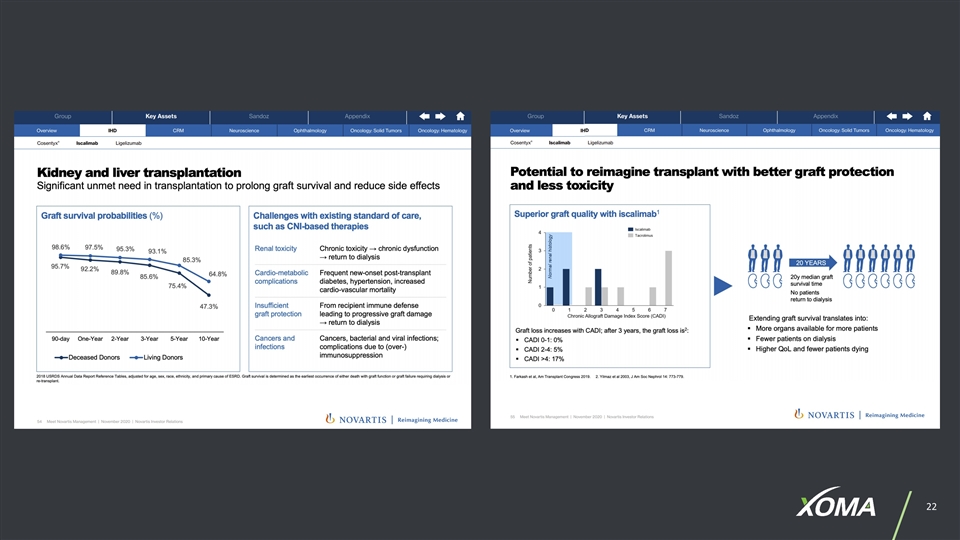
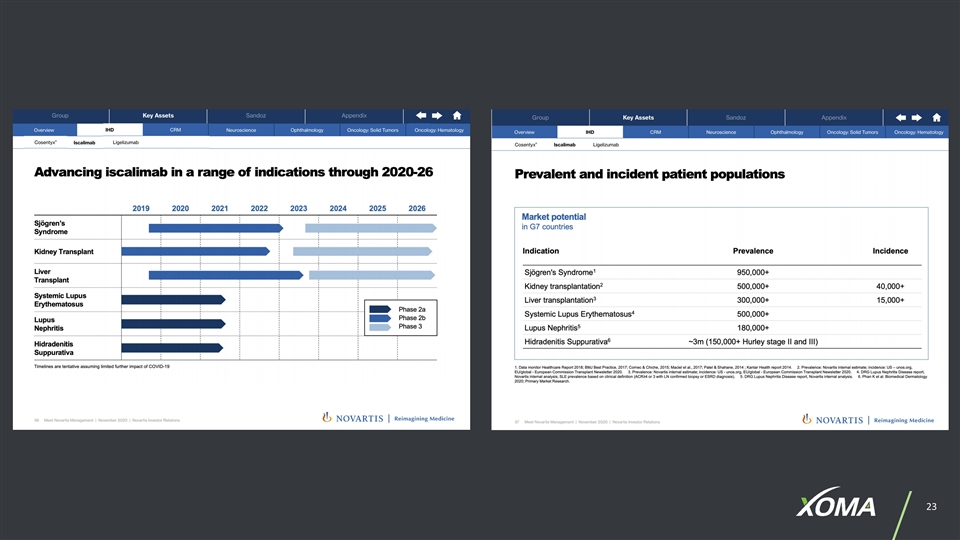
EXAMPLES OF CONDITIONS & DISEASES XOMA PARTNERS ARE PURSUING Lupus Nephritis Multiple Myeloma Squamous Cell Carcinoma Systemic Lupus Erythematosus Metastatic Solid Tumors Non-muscle Invasive Bladder Cancer Kidney Transplant Prostate Cancer Advanced Solid Tumors Liver Transplant Urothelial Cancer Glioblastoma Hidradenitis Suppurativa Acute Myeloid Leukemia Bladder Cancer Type 1 Diabetes Colorectal Cancer Thromboembolism Sjögren’s Syndrome Gastroesophageal Cancer Myelofibrosis Graves' Disease Renal Cancer Ulcerative Colitis Myasthenia Gravis Non-Hodgkin Lymphoma Generalized Myasthenia Gravis Rheumatoid Arthritis Triple-negative Breast Cancer Anti-Botulism Congenital Hyperinsulinism Non-small Cell Lung Cancer Asthma End Stage Renal Disease Pancreatic Cancer Lysosomal Storage Disorders
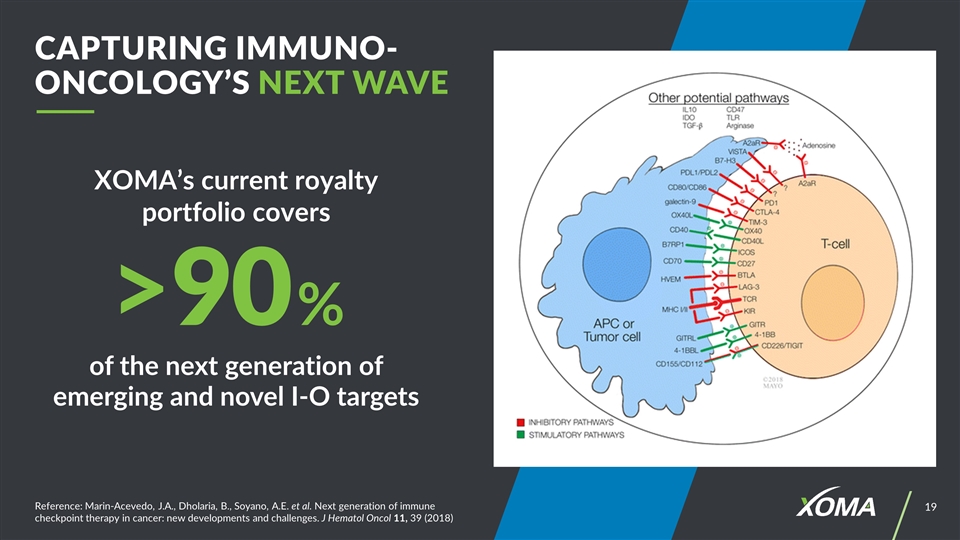
Reference: Marin-Acevedo, J.A., Dholaria, B., Soyano, A.E. et al. Next generation of immune checkpoint therapy in cancer: new developments and challenges. J Hematol Oncol 11, 39 (2018) XOMA’s current royalty portfolio covers Capturing Immuno-Oncology’s Next Wave of the next generation of emerging and novel I-O targets >90%
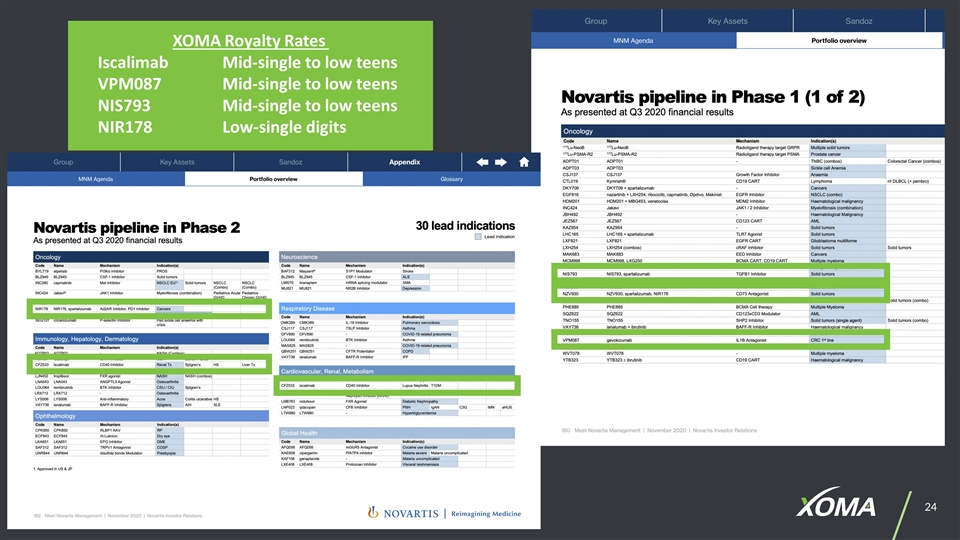
XOMA Royalty Rates IscalimabMid-single to low teens VPM087Mid-single to low teens NIS793Mid-single to low teens NIR178Low-single digits
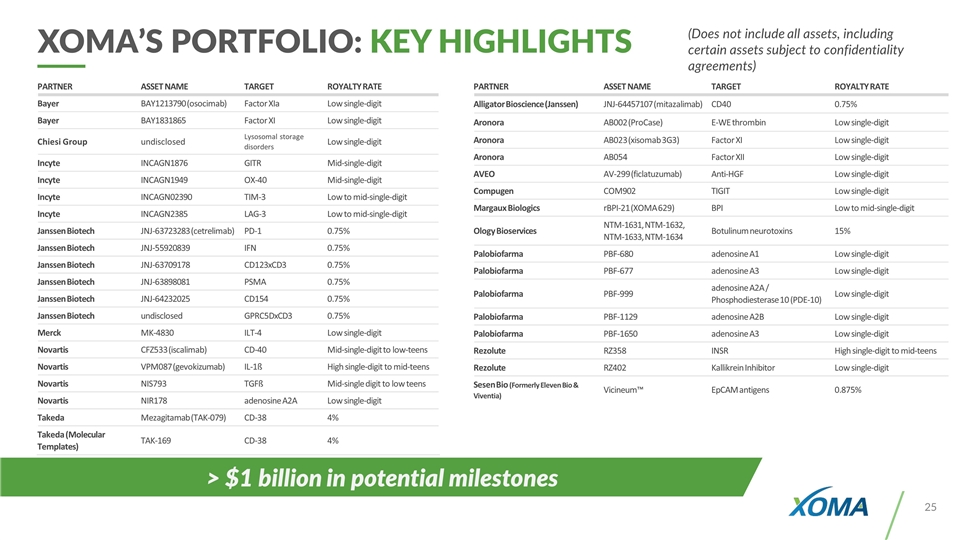
XOMA’S PORTFOLIO: KEY HIGHLIGHTS (Does not include all assets, including certain assets subject to confidentiality agreements) PARTNER ASSET NAME TARGET ROYALTY RATE Bayer BAY1213790 (osocimab) Factor XIa Low single-digit Bayer BAY1831865 Factor XI Low single-digit Chiesi Group undisclosed Lysosomal storage disorders Low single-digit Incyte INCAGN1876 GITR Mid-single-digit Incyte INCAGN1949 OX-40 Mid-single-digit Incyte INCAGN02390 TIM-3 Low to mid-single-digit Incyte INCAGN2385 LAG-3 Low to mid-single-digit Janssen Biotech JNJ-63723283 (cetrelimab) PD-1 0.75% Janssen Biotech JNJ-55920839 IFN 0.75% Janssen Biotech JNJ-63709178 CD123xCD3 0.75% Janssen Biotech JNJ-63898081 PSMA 0.75% Janssen Biotech JNJ-64232025 CD154 0.75% Janssen Biotech undisclosed GPRC5DxCD3 0.75% Merck MK-4830 ILT-4 Low single-digit Novartis CFZ533 (iscalimab) CD-40 Mid-single-digit to low-teens Novartis VPM087 (gevokizumab) IL-1ß High single-digit to mid-teens Novartis NIS793 TGFß Mid-single digit to low teens Novartis NIR178 adenosine A2A Low single-digit Takeda Mezagitamab (TAK-079) CD-38 4% Takeda (Molecular Templates) TAK-169 CD-38 4% PARTNER ASSET NAME TARGET ROYALTY RATE Alligator Bioscience (Janssen) JNJ-64457107 (mitazalimab) CD40 0.75% Aronora AB002 (ProCase) E-WE thrombin Low single-digit Aronora AB023 (xisomab 3G3) Factor XI Low single-digit Aronora AB054 Factor XII Low single-digit AVEO AV-299 (ficlatuzumab) Anti-HGF Low single-digit Compugen COM902 TIGIT Low single-digit Margaux Biologics rBPI-21 (XOMA 629) BPI Low to mid-single-digit Ology Bioservices NTM-1631, NTM-1632, NTM-1633, NTM-1634 Botulinum neurotoxins 15% Palobiofarma PBF-680 adenosine A1 Low single-digit Palobiofarma PBF-677 adenosine A3 Low single-digit Palobiofarma PBF-999 adenosine A2A / Phosphodiesterase 10 (PDE-10) Low single-digit Palobiofarma PBF-1129 adenosine A2B Low single-digit Palobiofarma PBF-1650 adenosine A3 Low single-digit Rezolute RZ358 INSR High single-digit to mid-teens Rezolute RZ402 Kallikrein Inhibitor Low single-digit Sesen Bio (Formerly Eleven Bio & Viventia) Vicineum™ EpCAM antigens 0.875% > $1 billion in potential milestones
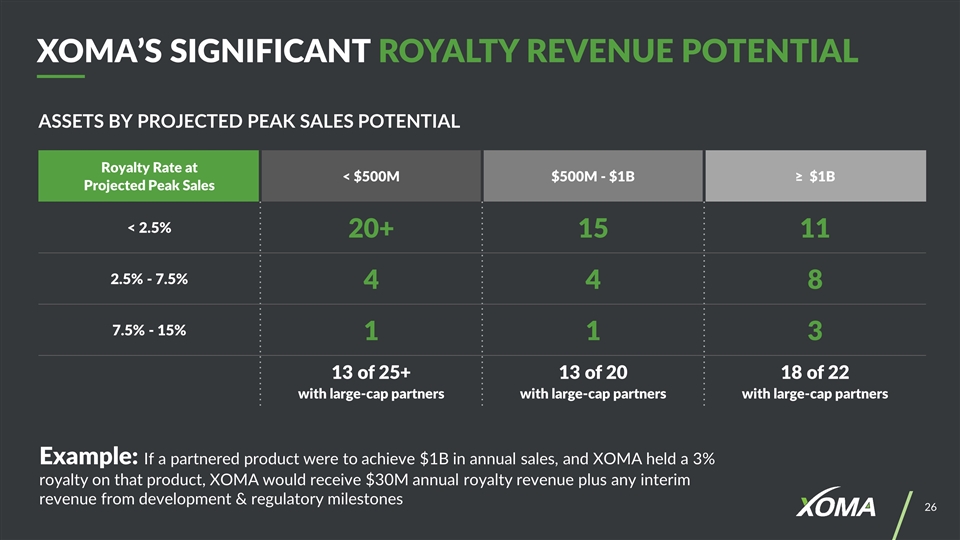
ASSETS BY PROJECTED PEAK SALES POTENTIAL XOMA’S SIGNIFICANT ROYALTY REVENUE POTENTIAL Example: If a partnered product were to achieve $1B in annual sales, and XOMA held a 3% royalty on that product, XOMA would receive $30M annual royalty revenue plus any interim revenue from development & regulatory milestones Royalty Rate at Projected Peak Sales < $500M $500M - $1B ≥ $1B < 2.5% 20+ 15 11 2.5% - 7.5% 4 4 8 7.5% - 15% 1 1 3 13 of 25+ with large-cap partners 13 of 20 with large-cap partners 18 of 22 with large-cap partners
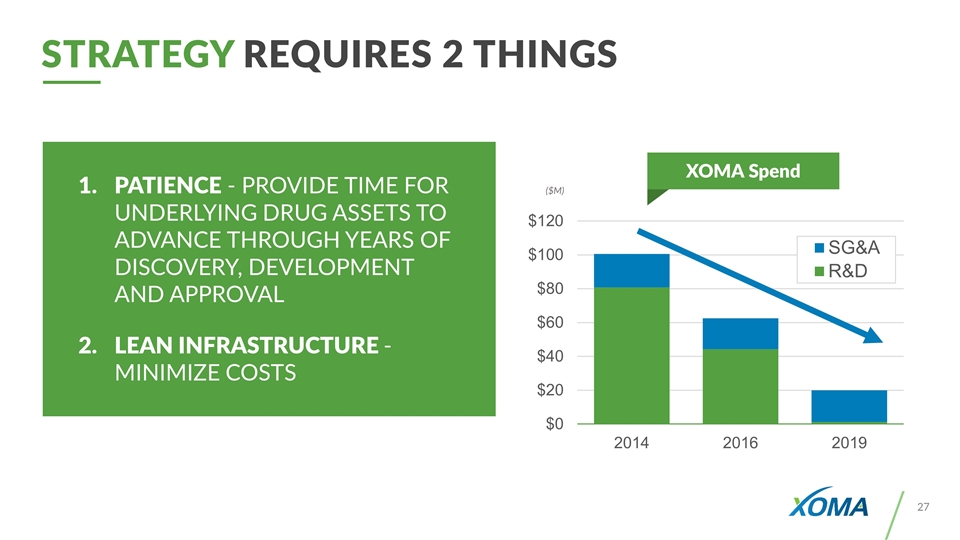
STRATEGY Requires 2 Things XOMA Spend ($M) PATIENCE - PROVIDE TIME FOR UNDERLYING DRUG ASSETS TO ADVANCE THROUGH YEARS OF DISCOVERY, DEVELOPMENT AND APPROVAL LEAN INFRASTRUCTURE - MINIMIZE COSTS

Partner-driven financial events $25M from Novartis, blend of cash and debt reduction Accelerated $1.4M in payments from Rezolute $2M milestone from Takeda, $1M from Merck Increased number of royalty licenses by >40% since 3Q18 Acquired milestone & royalty interests in 7 partnered assets, 8 unpartnered assets, future assets from 2 technology platforms Licensed XOMA’s IL-2 mAb to Zydus for development and commercialization rights in India, Mexico, and Brazil Added Natasha A. Hernday to Board of Directors Novartis Dosed NIS793 (anti-TGFβ mAb) in first metastatic pancreatic cancer patient – launch of Phase 2 development Launched gevokizumab development in oncology Conducting multiple iscalimab (CFZ533) Phase 2 trials Takeda Launched mezagitamab (TAK-079) Phase 2 program -myasthenia gravis and thrombocytopenia studies Merck Commenced MK-4830 Phase 2 development with NSCLC study Bayer Initiated Phase 2 osocimab (BAY1213790) study in kidney failure setting Sesen Bio & Vicineum™ for the treatment of BCG-unresponsive non-muscle invasive bladder cancer Initiated rolling BLA in Dec 2019 Ology Bioservices awarded DoD contract to advance anti- botulinum neurotoxin monoclonal antibodies RECENT HIGHLIGHTS OPERATIONAL PARTNERS & PARTNERED ASSETS

Looking ahead Acquire additional milestone and royalty interest assets to continue to grow the portfolio Maintain lean cost infrastructure and financial discipline Current balance sheet sufficient to fund operations for multiple years ~$1M per month core G&A expense OPERATIONAL NOVARTIS Iscalimab data readouts – multiple Phase 2 studies NOVARTIS Gevokizumab advancing to Phase 2 SESEN BIO Completed BLA Filing / PDUFA date Study Initiations & Results Data Presentations / Publications PARTNERS & PARTNERED ASSETS

XOMA holds 65+ current assets; pharmaceutical partners fund research & development and cover 100% of costs XOMA sources royalty rights through deep industry network XOMA constructs an increasingly diverse and expanding portfolio to increase odds of success and mitigate binary risk XOMA has low-cost infrastructure; future potential revenues largely fall to bottom line Why XOMA’s portfolio IS valuable